Qualification Services
With You Every Step Of The Way
In order to comply with EN 1422 - EN ISO 11135, ISO 13485 Standards, and GMP guidelines, our protocols and procedures are created to help you comply with validation requirements and meet your requirement specifications.
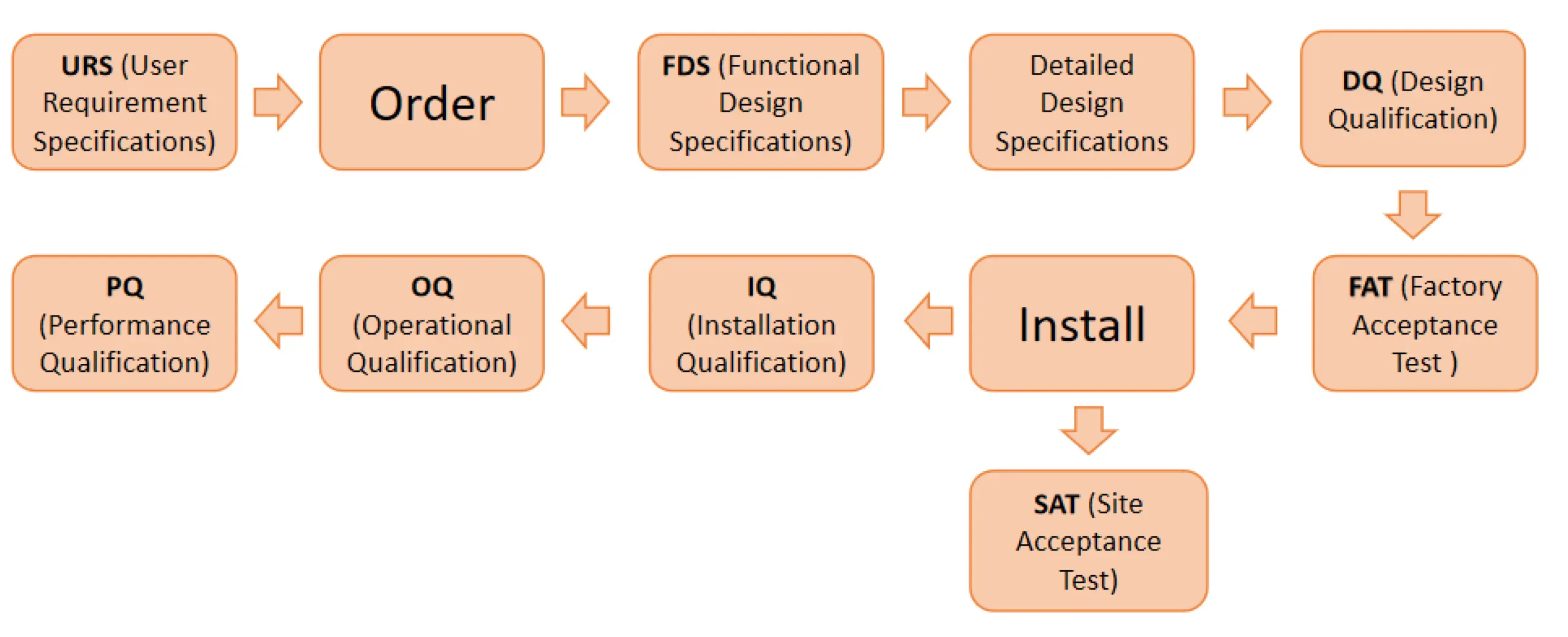
URS (User Requirement Specifications)
Definition of requirements to fulfill the demands of the process. Generally, URS is required for eto sterilizer machine before making the design.
In order to meet the user requirement, we pay special attention to the URS (User Requirement Specifications) (such as chamber size, product, package, EO percentage, etc.)
Issued by: User
Click to download the URS sheet and let us know your request.
 DOWNLOAD URS
DOWNLOAD URSFDS (Functional Design Specifications)
Specification in which the demands of the buyer are transferred into a technical solution (from the manufacturer's point of view)
Issued by: Manufacturer
DESIGN SPECIFICATION (DS)
Specification of the technical basis and specification of the limiting factors that are to be considered during development, construction and assembly. DS ensures that all customer requirements and current valid standards from CGMP, EU, EN ISO and the FDA are satisfied.
FAT / SAT (Factory Acceptance Test /Site Acceptance Test)
Documented acceptance tests at the supplier/customer with test log, test summary, list of open issues, and release certificates.
In BOCON, before the sale, the factory provides customers with the design and consultation on the overall equipment and facilities of the ETO workshop and assists in the formulation of the overall planning and layout of the ETO workshop. According to the characteristics of the customer site and product, material, packaging, transportation, and future development, we choose the material, configuration, control mode, volume, and quantity for the sterilizer. To assist in the preparation of the ethylene oxide sterilization process, verification scheme, ETO workshop management rules and regulations, equipment use and maintenance, etc. At the same time to provides relevant standards, preheating, residual analysis, processing technology consultation, and cooperation.
DQ, IQ, OQ, PQ
DQ(Design Qualification)
IQ (Installation Qualification)
OQ(Operational Qualification)
PQ(Performance Qualification)
From product and requirements specifications, to risk analyses, to planning and execution of the individual qualification steps, we takes care of everything implementation requires.
Validation: BOCON would provide software validation. IQ, OQ report, and support of PQ after installation.